Lyme Disease Market Growth Highlighted by Pipeline Advances and Expanding Patient Population | DelveInsight
According to DelveInsight’s analysis, the market for Lyme disease is anticipated to increase during the forecast period (2025–2034), owing to the launch of emerging therapies such as Valneva and Pfizer’s VLA15 and Tarsus Pharmaceuticals’ TP-05, Moderna’s mRNA-1982 and mRNA-1975, and others, and healthcare spending globally.
New York, USA, Oct. 01, 2025 (GLOBE NEWSWIRE) -- Lyme Disease Market Growth Highlighted by Pipeline Advances and Expanding Patient Population | DelveInsight
According to DelveInsight’s analysis, the market for Lyme disease is anticipated to increase during the forecast period (2025–2034), owing to the launch of emerging therapies such as Valneva and Pfizer’s VLA15 and Tarsus Pharmaceuticals’ TP-05, Moderna’s mRNA-1982 and mRNA-1975, and others, and healthcare spending globally.
DelveInsight’s Lyme Disease Market Insights report includes a comprehensive understanding of current treatment practices, emerging Lyme disease drugs, market share of individual therapies, and current and forecasted Lyme disease market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
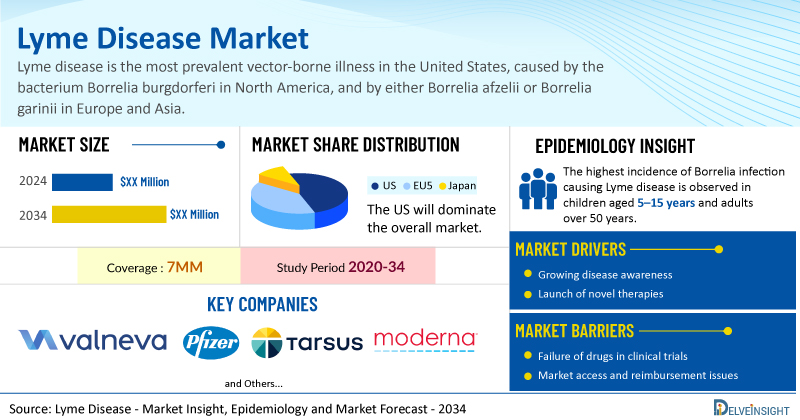
Lyme Disease Market Summary
- The total Lyme disease treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of Lyme disease, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- The highest incidence of Borrelia infection causing Lyme disease is observed in children aged 5–15 years and adults over 50 years.
- Key Lyme disease companies, including Valneva, Pfizer, Tarsus Pharmaceuticals, Moderna, and others, are actively working on innovative Lyme disease drugs.
- Some of the key Lyme disease therapies in clinical trials include VLA15, TP-05, mRNA-1982, mRNA-1975, and others. These novel Lyme disease therapies are anticipated to enter the Lyme disease market in the forecast period and are expected to change the market.
Discover which Lyme disease medications are expected to grab the market share @ Lyme Disease Market Report
Key Factors Driving the Growth of the Lyme Disease Market
Rising Case Numbers and Expanded Surveillance
The increasing number of reported cases and broader surveillance are driving demand for diagnostics, treatments, and prevention tools, thereby fueling the growth of the Lyme disease market across testing, therapeutics, and public health services.
Growing Diagnostics Market
Demand for more accurate, faster, and higher-volume testing (ELISA, modified two-tier algorithms, PCR, point-of-care assays) is pushing market expansion and investment in new platforms. Recent market analyses show multi-billion-dollar global testing markets and mid-single-digit to low-double-digit CAGRs.
Launch of Emerging Lyme Disease Therapies
Companies worldwide are diligently working to develop novel therapies, achieving considerable success over the years. Key players, such as Valneva/Pfizer (VLA15), Tarsus Pharmaceuticals (TP-05), Moderna (mRNA-1982 and mRNA-1975), and others, are developing therapies for the management of Lyme disease.
Lyme Disease Market Analysis
The approach to treating Lyme disease depends on the stage of the illness, as the underlying pathophysiology differs at each stage. Consequently, antibiotic therapy must be tailored to the specific clinical manifestations. Current guidelines and recommendations follow this stage-specific strategy to help select the most appropriate antibiotics for each situation.
In 1998, the FDA approved LYMERIX (GSK), a recombinant Osp-A–based vaccine designed to prevent Lyme disease in adults. The vaccine required three doses administered over two tick seasons and demonstrated approximately 76% efficacy in preventing Lyme disease after completion of the whole series. LYMERIX and other Osp-A vaccines work by producing antibodies that block the transmission of Borreliella spirochetes from infected ticks during feeding on humans. However, public acceptance was low due to several factors, and in 2002, Smith-Kline Beecham withdrew LYMERIX from the market. Subsequent attempts by companies such as MedImmune, Baxter, and Connaught Laboratories to develop Lyme vaccines were also discontinued, leading to a decline in industry interest.
Two decades after LYMERIX, renewed efforts are underway to create next-generation human vaccines for Lyme disease prevention. A significant challenge for these vaccines is addressing the genetic diversity of pathogenic Borreliella species and strains in different regions. To overcome this, current vaccine candidates are designed to include multiple immunogenic antigens or several serotypes of a single antigen to broaden protection.
Learn more about the Lyme disease treatment options @ Lyme Disease Treatment Market
Lyme Disease Competitive Landscape
Key emerging therapies for Lyme disease include VLA15 (Valneva and Pfizer), TP-05 (Tarsus Pharmaceuticals, Inc.), mRNA-1982 and mRNA-1975 (Moderna), and others.
Valneva and Pfizer’s VLA15 is an experimental, multivalent recombinant protein vaccine designed to target six Borrelia serotypes commonly associated with Lyme disease in the United States and Europe. It works by stimulating the production of antibodies against outer surface protein A (OspA), which the bacterium expresses on its outer surface within ticks; by blocking OspA, the vaccine prevents transmission to humans. VLA15 is currently the most advanced Lyme disease vaccine candidate in clinical development. Pfizer, in collaboration with Valneva, aims to submit a Biologics License Application (BLA) to the FDA and a Marketing Authorization Application (MAA) to the EMA in 2026, contingent on favorable trial results. The vaccine received FDA Fast Track Designation in 2017.
Tarsus Pharmaceuticals’ TP-05 is an oral systemic formulation of lotilaner being developed to prevent Lyme disease. It is believed to be the only non-vaccine, drug-based preventive therapy in development that targets infected tick vectors and eliminates them before they can transmit Borrelia bacteria. TP-05 is designed to quickly achieve therapeutic systemic levels of lotilaner, a well-established anti-parasitic agent, offering on-demand protection. It is currently undergoing a Phase IIa trial as per the company’s pipeline.
Moderna has introduced two new mRNA vaccine candidates for Lyme disease: mRNA-1982 and mRNA-1975. The mRNA-1982 candidate is intended to elicit antibodies against Borrelia burgdorferi, the primary cause of Lyme disease in the US. Meanwhile, mRNA-1975 is designed to trigger antibody production against the four main Borrelia species responsible for Lyme disease in both the US and Europe.
The anticipated launch of these emerging Lyme disease therapies are poised to transform the Lyme disease market landscape in the coming years. As these cutting-edge Lyme disease therapies continue to mature and gain regulatory approval, they are expected to reshape the Lyme disease market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for Lyme disease, visit @ Lyme Disease Medication
What is Lyme Disease?
Lyme disease is the most prevalent vector-borne illness in the United States, caused by the bacterium Borrelia burgdorferi in North America, and by either Borrelia afzelii or Borrelia garinii in Europe and Asia. The infection is transmitted through bites from infected black-legged ticks (Ixodes species) and typically begins with early localized symptoms such as erythema migrans and flu-like signs. If untreated, it can progress to disseminated or late-stage disease, potentially impacting the joints, nervous system, or heart. Diagnosis is based on clinical presentation, history of tick exposure, and serologic tests. Early treatment with antibiotics such as doxycycline, amoxicillin, or cefuroxime is effective in preventing long-term complications. While most patients recover completely, delayed or insufficient treatment can lead to persistent symptoms. Preventive measures focus on reducing tick exposure through the use of protective clothing, insect repellents, prompt tick removal, and managing tick habitats.
Lyme Disease Epidemiology Segmentation
The Lyme disease epidemiology section provides insights into the historical and current Lyme disease patient pool and forecasted trends for the leading markets. According to the Centers for Disease Control and Prevention, approximately 476K Americans are diagnosed and treated for Lyme disease each year, with at least a further 130K cases in Europe.
The Lyme disease market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Incident cases of Lyme Disease
- Gender-specific incident cases of Lyme Disease
- Age-specific incident cases of Lyme Disease
- Treatable cases of Lyme Disease
Download the report to understand Lyme disease management @ Lyme Disease Treatment Options
| Lyme Disease Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Lyme Disease Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Lyme Disease Epidemiology Segmentation | Incident cases of Lyme Disease, Gender-specific incident cases of Lyme Disease, Age-specific incident cases of Lyme Disease, and Treatable cases of Lyme Disease |
| Key Lyme Disease Companies | Valneva, Pfizer, Tarsus Pharmaceuticals, Moderna, and others |
| Key Lyme Disease Therapies | VLA15, TP-05, mRNA-1982, mRNA-1975, and others |
Scope of the Lyme Disease Market Report
- Lyme Disease Therapeutic Assessment: Lyme Disease current marketed and emerging therapies
- Lyme Disease Market Dynamics: Conjoint Analysis of Emerging Lyme Disease Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Lyme Disease Market Unmet Needs, KOL’s views, Analyst’s views, Lyme Disease Market Access and Reimbursement
Discover more about Lyme disease drugs in development @ Lyme Disease Clinical Trials
Table of Contents
| 1 | Lyme Disease Market Key Insights |
| 2 | Lyme Disease Market Report Introduction |
| 3 | Executive Summary of Lyme Disease |
| 4 | Key Events of Lyme Disease |
| 5 | Epidemiology and Market Forecast Methodology |
| 6 | Lyme Disease: Market Overview at a Glance |
| 6.1 | Total Market Share (%) Distribution of Lyme Disease by Therapies in 2024 |
| 6.2 | Total Market Share (%) Distribution of Lyme Disease by Therapies in 2034 |
| 7 | Lyme Disease: Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Lyme Disease Signs and Symptoms |
| 7.3 | Lyme Disease Causes |
| 7.4 | Lyme Disease Mechanism of Action |
| 7.5 | Lyme Disease Diagnosis |
| 7.6 | Lyme Disease Treatment |
| 8 | Patient Journey of Lyme Disease |
| 9 | Epidemiology and Patient Population of Lyme Disease |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale: The 7MM |
| 9.3 | Total Incident Cases of Lyme Disease in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Incident Cases of Lyme Disease in the United States |
| 9.4.2 | Gender-specific Incident Cases of Lyme Disease in the United States |
| 9.4.3 | Age-specific Incident Cases of Lyme Disease in the United States |
| 9.4.4 | Treatable Cases of Lyme Disease in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| List to be continued in the report… | |
| 10 | Emerging Lyme Disease Drugs |
| 10.1 | Key Competitors |
| 10.2 | TP-05: Tarsus Pharmaceuticals |
| 10.2.1 | Product Description |
| 10.2.2 | Other Developmental Activities |
| 10.2.3 | Clinical Development |
| 10.2.3.1 | Clinical Trials Information |
| 10.2.4 | Safety and Efficacy |
| 10.2.5 | Analyst Views |
| List to be continued in the report… | |
| 11 | Lyme Disease Market: Seven Major Market Analysis |
| 11.1 | Key Findings |
| 11.2 | Key Market Forecast Assumptions |
| 11.3 | Lyme Disease Market Outlook |
| 11.4 | Conjoint Analysis |
| 11.5 | Total Market Size of Lyme Disease in the 7MM |
| 11.6 | Total Market Size of Lyme Disease by Therapies in the 7MM |
| 11.7 | Market Size of Lyme Disease in the US |
| 11.7.1 | Total Market Size of Lyme Disease |
| 11.7.2 | The Market Size of Lyme Disease by Therapies |
| 11.8 | Market Size of Lyme Disease in EU4 and the UK |
| 11.9 | Market Size of Lyme Disease in Japan |
| 12 | Unmet Needs of Lyme Disease |
| 13 | SWOT Analysis of Lyme Disease |
| 14 | KOL Views of Lyme Disease |
| 15 | Market Access and Reimbursement of Lyme Disease |
| 16 | Bibliography |
| 17 | Lyme Disease Market Report Methodology |
Related Reports
Lyme Disease Clinical Trial Analysis
Lyme Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Lyme Disease companies, including Valneva, Cortene, Blue Lake Biotechnology, Tarsus Pharmaceuticals, Inovio Pharmaceuticals, Aegis Life, Abzyme Therapeutics, among others.
Rosacea Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Rosacea companies, including Sol-Gel Technologies, Galderma, AiViva BioPharma, Tarsus Pharmaceuticals, Aobiome Therapeutics, among others.
Rosacea Clinical Trial Analysis
Rosacea Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Rosacea companies, including Sol-Gel Technologies, Maruho Co., Ltd., AOBiome LLC, CAGE Bio Inc., Dermata Therapeutics, BioMimetix JV, LLC, Dr Reddy's Laboratories, Amgen, Alfasigma, AiViva BioPharma, BioPharmX, Inc., Botanix Pharmaceuticals, Evommune, Tarus Pharmaceuticals, Cellix Bio., Fortress Biotech, AOBiome, among others.
Bacteremia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key bacteremia companies, including Merck & Co., Inc., Ronak Daru Co., Bayer AG, Pfizer Inc., Baxter, ANI Pharmaceuticals, Inc., Theravance Biopharma, Novartis AG, Fresenius SE & Co. KGaA, Mylan N.V., Sun Pharmaceutical Industries Ltd., among others.
Bacteremia Clinical Trial Analysis
Bacteremia Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key bacteremia companies, including XBiotech, LegoChem Biosciences, Basilea Pharmaceutica, ContraFect, Merck & Co., Cumberland Pharmaceuticals, Theravance Biopharma, Entasis Therapeutics, Melinta Therapeutics, GlaxoSmithKline, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
